Invasive Crayfish 101
Diversity

The eastern United States harbors more than 60% of the world’s currently recognized crayfish species, making it a global ‘hotspot’ for crayfish biodiversity (Richman et al., 2015). The United States and Canadian provinces surrounding the Laurentian Great Lakes are home to approximately 40 crayfish species (Taylor et al., 2015), and a moderate number of these species are rare or have narrow natural ranges (Page, 1985; Taylor et al., 2015; Richman et al., 2015). In the United States and Canada, crayfish species richness generally increases moving south and east. Some states in the southern-most areas of the Great Lakes Region such as Illinois, Indiana, and Ohio have numerous crayfish species with narrowly endemic ranges – the sinkhole crayfish (Faxonius theaphionensis) in central Indiana and the depression crayfish (Cambarus rusticiformes) in southern Illinois are just two examples.

Many crayfish species in the Great Lakes Region are only known to occur in streams and rivers, but some species can persist, or even thrive, in lentic waters such as ponds, natural lakes, or reservoirs. An even smaller number of these crayfishes are almost completely terrestrial – they spend most of their lives in underground chambers, removed from direct contact with permanent waterbodies. Unfortunately, non-indigenous crayfish species introduced through human activities present a significant threat to many of the native crayfish species in the Great Lakes and surrounding areas. In some cases, these non-indigenous crayfishes can be considered invasive, given their abilities to rapidly colonize new habitats and displace native species. Invasive crayfishes have already displaced native crayfishes from considerable portions of their ranges and have dramatically altered ecosystem structure in some cases (Wilson et al., 2004). Invasive crayfishes are therefore a formidable threat to both crayfish biodiversity and freshwater ecosystems in the Great Lakes and worldwide (Lodge et al., 2000a).
Ecological Significance

As Prey
Crayfishes serve as prey for over 200 animal species, including mammals, birds, reptiles, amphibians, fishes, and insects (DiStefano, 2005). Among aquatic predators, the fish family Centrarchidae, including many popular sport fishes such as smallmouth bass (Micropterus dolomieu) and rock bass (Ambloplites rupestris), are particularly prominent crayfish predators (Probst et al., 1984; Rabeni, 1992). A wide variety of other fish species, ranging from brook trout (Salvelinus fontinalis) to creek chubs (Semotilus atromaculatus) have also been shown to allocate portions of their diet to the consumption of crayfishes (Newsome and Gee, 1978; Gowing and Momot, 1979). Crayfishes also represent a vital food source for other aquatic taxa, such as the federally endangered aquatic salamander known as the hellbender (Cryptobranchus alleganiensis; Wiggs, 1976). Beyond aquatic ecosystems, crayfishes are also frequently consumed by a variety of terrestrial animals, including minks, raccoons, and even some wading birds (Baker et al., 1945; Toweill, 1974; Martin and Hamilton, 1985).

As Consumers
Crayfishes are generally thought to be omnivores and have been shown to consume a wide variety of food items, ranging from phytoplankton to fish. In terms of primary producers, crayfishes consume filamentous algae (Goldman, 1973) and aquatic plants (Creed, 1994). Some crayfishes can consume so much algal and plant material that they can strongly influence the population densities of these organisms (Goldman, 1973). Crayfishes also function as important predators, consuming a variety of vertebrate and invertebrate prey. Crayfishes feed on many different types of invertebrate prey, including snails (Kreps et al., 2012), insects and their larvae (Parkyn et al., 2001), and even other crayfishes (Nakata & Goshima, 2006). Beyond invertebrates, crayfishes can consume vertebrates such as fishes (Rahel and Stein, 1988) and amphibians, particularly their eggs or larvae (Axelsson et al., 1997).
As Ecosystem Engineers
Crayfishes live in a variety of habitat types, both aquatic and terrestrial, and they can have remarkable impacts on the ecosystems in which they live. Crayfishes can both directly and indirectly alter habitat quality and resource availability for other organisms, often more so than other co-occurring taxa (Reynolds et al., 2013). One mechanism by which crayfishes can accomplish this is through their burrowing behavior. Crayfishes are generally adept in constructing burrows and other subterranean chambers in aquatic, and sometimes terrestrial, habitats. These burrowing abilities allow them to substantially modify and create interstitial spaces within substrates, creating habitats that can be utilized by other organisms (Creed and Reed, 2004).
The same burrowing behaviors can also significantly alter sediment erosion rates in some ecosystems (Statzner et al., 2000; 2003). In terrestrial habitats, primary burrowing crayfishes (those species that spend most – or all – of their adult lives away from permanent bodies of water) construct complex networks of tunnels and chambers deep into the soil. These often expansive subterranean networks can serve as critical conduits for water or gas exchange, oxygenating and draining otherwise poor soils (Richardson, 1983; 2007). Crayfishes can also consume staggering amounts of detritus, primarily in the form of leaf litter and other decaying plant material (Huryn and Wallace, 1987; Schofield et al., 2001). In fact, detrital processing by crayfishes can strongly alter the abundances of insect larvae, such as heptageniid mayflies (Creed and Reed, 2004), which can be an important food source for fishes and other stream vertebrates (Hoopes, 1960).
Life History
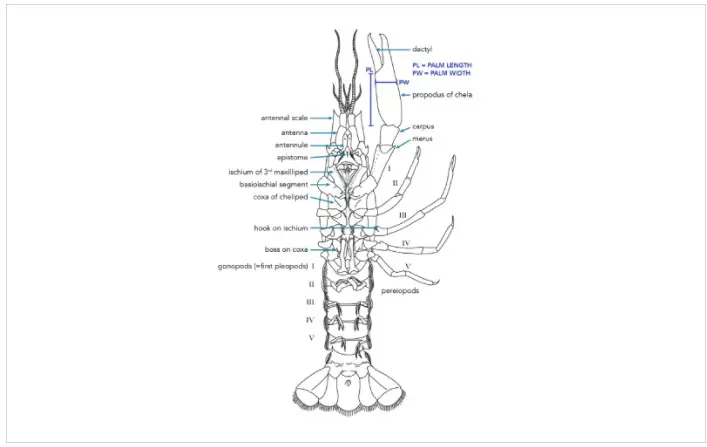
Crayfishes are born from fertilized eggs, which are extruded by a gravid female and subsequently attached to the swimmerettes (pleopods) on her abdomen. This process occurs during the spring months, usually between March and June (Taylor et al., 2015), in most North American crayfishes. The fertilized eggs usually hatch within a few days of oviposition, although the newly-hatched, juvenile crayfish stay attached to their mother for a few more days in order to increase their body size. Once large enough, the juvenile crayfish detach from their mother’s abdomen and become functional, free-living individuals. Juvenile crayfishes spend the warm summer months foraging for food in order to grow as much as possible before food becomes limited during the cooler fall and winter months. Juvenile crayfishes grow rapidly during their first few months of life, and as a result, they molt frequently during this period.
All crayfishes have a calcified exoskeleton that is somewhat hard and inhibits their growth. Therefore, they will periodically exude this exoskeleton in order to grow more rapidly. Freshly molted crayfishes are quite soft (and vulnerable to predation), but this allows them to grow quickly before their new exoskeleton calcifies. Juvenile crayfishes are often recruited to the adult population the following spring, although there is evidence in some species that individuals can become reproductively active at the end of their first summer (Pflieger, 1996). Once adulthood is reached, molting usually only occurs once or twice each year in most North American species (Taylor et al., 2015).
The timing of the adult molt cycle often corresponds to the annual reproductive cycle. Male crayfishes in the family Cambaridae (most North American crayfishes) exhibit cyclic dimorphism with respect to their reproductive morphology. Most notably, the gonopods (sperm transfer organs) alternate between a reproductive (late fall-early spring) and a non-reproductive (late spring-early fall) form, with the reproductive forms often much more slender and with more pointed tips. Males in this reproductive state are often referred to as ‘form I’, and such specimens are often the basis of taxonomic identifications. There is also some evidence to suggest that female Cambarid crayfishes exhibit similar patterns of cyclic dimorphism, with respect to the width of their abdomen (wider in reproductive females) and the presence of glair, the glue-like substance used to adhere fertilized eggs to the abdomen (Wetzel, 2002).
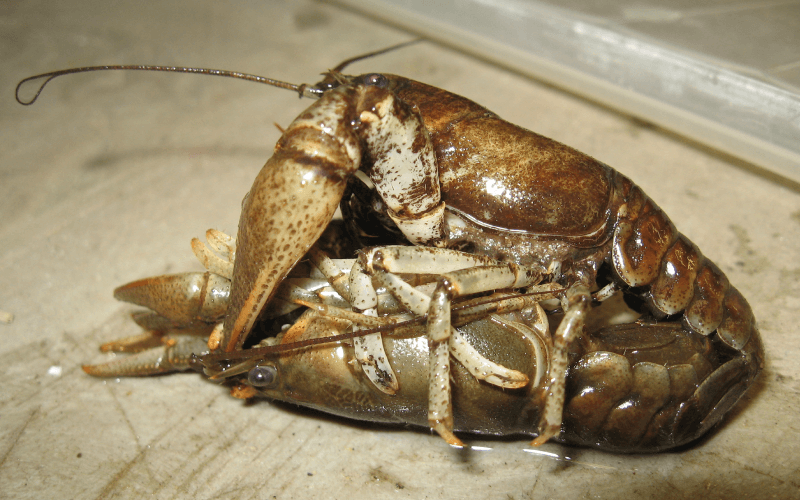
Copulation occurs during the fall and winter months in many species (Taylor et al., 2015), while the summer months may be primarily used for foraging and growing. Most crayfish species in the Great Lakes and the broader Midwest region are thought to live approximately 2-3 years (Taylor et al., 2015). However, some species, such as those in the genus Cambarellus, may live for less than a year, while others, such as those in the genus Cambarus, may live closer to five or more years (DiStefano et al., 2016).
Species of Concern
The invasive rusty and red swamp crayfishes are currently species of primary concern for the Great Lakes region. Ongoing work of the ICC involves the identification of additional species that may prove to be a threat to the region.
Rusty Crayfish (Faxonius rusticus)
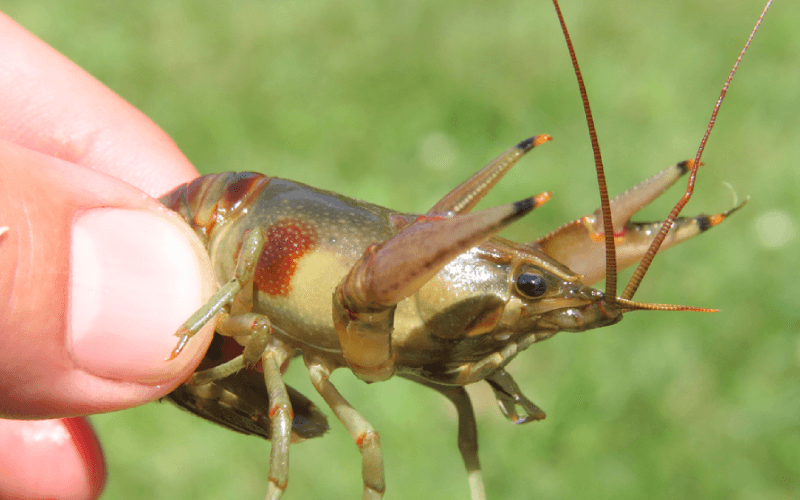
Identification: Rusty crayfish are typically olive-green to gray in body color, with larger individuals sometimes possessing chelae (claws) with light blue or violet hues and black rings around the tips of the orange dactyls (fingers). Note—some other species also have black rings around the tips of the dactyls. Most notably, rusty crayfish have two unique 'rust' or brown-colored spots on each side of the posterior margin of their carapace (cephalothorax). No other crayfish species in the Great Lakes region possess these unique spots on the carapace.
Spread: Rusty crayfish are native to portions of Indiana and Ohio, although they have expanded their range dramatically to now include most of the upper Midwest, including portions of Canada. Isolated, introduced populations now exist as far west as Oregon and as far east as Maine. Rusty crayfish may have expanded their range due in part to the artificial connection of waterways (via canals) in the upper Midwest and Great Lakes regions throughout the 19th and 20th centuries (Creaser, 1931), and sporadic anthropogenic translocations and introductions may have further facilitated their rapid range expansion. Rusty crayfish are also tolerant to a wide variety of habitat conditions, and they appear to survive well in many habitat types, including lakes, ponds, streams, and rivers (Taylor and Redmer, 1996). In addition to their apparent adaptability, rusty crayfish also grow larger and can be more aggressive than similar congeneric species (Roth and Mitchell, 2005), which may partially explain their ability to displace some native crayfishes.
Distribution: Native to the Ohio River basin and parts of Kentucky, Indiana, Tennessee, and Illinois, their range has expanded and now includes all the Great Lakes states, parts of New England, mid-Atlantic states, and parts of Ontario, Canada. It is also present in several western states, including Wyoming, Colorado, Nevada, and Oregon.
Red Swamp Crayfish (Procambarus clarkii)

Identification: Adult red swamp crayfish can be easily identified by their brick red (although sometimes lighter or darker) body coloration, but juvenile red swamp crayfish can be seen in a variety of tan or brown shades and can be freckled with small, dark spots of pigmentation. In addition to their unique coloration, red swamp crayfish differ from most other crayfish species found in the Great Lakes region by possessing chelae (claws) that are long, slender, and contain multiple small tubercles on the dorsal surface. These tubercles often extend onto the carapace (cephalothorax). The native white river crayfish (Procambarus acutus) can be easily confused with the red swamp crayfish, although the red swamp crayfish has a closed areola, meaning that the two lines on the dorsal surface of its carapace are touching in the middle. Conversely, white river crayfish possess an open areola, meaning that these lines are always visibly separated. Note—alternate color morphs of red swamp crayfish exist in the pet trade, usually consisting of blue or orange hues.
Spread: Red swamp crayfish are among the most popular and frequently cultured crayfish species in the world, and they are commonly encountered in the bait, aquaculture, and pet trade industries (Taylor et al., 2015). This may be due in part to their popularity as a food item in the southern United States, or because of their relative ability to tolerate a variety of different habitat types and conditions. There are also unique color morphs of the species that can be encountered in the pet trade, which make red swamp crayfish a popular species with aquarium hobbyists. Red swamp crayfish have been translocated and introduced around the world, with many isolated populations existing in the Great Lakes and surrounding areas. Red swamp crayfish have been particularly detrimental in Europe, where they have contributed to the extirpation and decline of multiple native European crayfishes by exposing these crayfishes to crayfish plague, a highly infectious disease.
Distribution: Native to the south-central United States along the Gulf Coast and along the Mississippi River basin as far north as southern Illinois, the red swamp crayfish is now in several of the other Great Lakes, mid-Atlantic and far western states. Populations have also been reported from Idaho, Utah, Arizona, South Dakota, Nebraska, and Georgia.
Marbled Crayfish (Procambarus virginalis)

Identification: Marbled crayfish, also known as Marmorkrebs, get their name from their appearance. They have a marbled pattern that covers their entire back and claws and usually appear olive to brown in color when found in the wild. A dark stripe runs down each side of their carapace and abdomen. Male marbled crayfish do not exist- all individuals are female and reproduce by parthenogenesis. Marbled crayfish are directly descended from the slough crayfish (Procambarus fallax) and have no known morphological differences between female slough crayfish and marbled crayfish. Any males can be assumed to be a slough crayfish.
Spread: The marbled crayfish is one of the most widely distributed species of crayfish in the pet trade (Faulkes, 2015). It was first discovered in the German aquarium trade in the mid-1990s and has since become established in many locations across the country. It has become established in other European countries, as well as Madagascar, Taiwan, and possibly Israel. They are a popular and easily accessible aquarium animal but there is little documentation of impacts beyond anecdotal information and laboratory studies (USFWS, 2023). Due to its parthenogenetic reproduction strategy, only one individual is needed to establish a population, making its potential invasiveness extremely high (Scholtz et al., 2003). In 2023, marbled crayfish was found in the wild in the Burlington, Ontario area, the first wild discovery of the species in the country.
Distribution: Marbled crayfish are the result of a mutation in captive slough crayfish and therefore have no natural populations (Martin et al, 2010). They have not been established or introduced in the United States, but multiple individuals have been found in the Lake Ontario drainage near Toronto.
Signal Crayfish (Pacifastacus leniusculus)


Identification: Signal crayfish are typically bluish-brown to reddish-brown in color on their dorsal side. The undersides of its claws are bright red, and the base of each claw joint has a white or turquoise colored patch. The surface of the claws and carapace are smooth and lack the pronounced bumps that are typical of other non-native crayfish. Their average carapace length is 50-70mm. The species is divided into three subspecies: leniusculus, klamathensis, and trowbridgii (Miller, 1960).
Spread: Potential introduction pathways for signal crayfish include live bait release, stocking for harvest, or stocking for fish food sources (Lodge et al., 2000b). Records show that this species was stocked in the Sacramento River and coastal waterways of California as early as 1912 (Riegel, 1959). The first confirmation of signal crayfish in the midwestern region of the US was made in Lake Winona, Minnesota in October of 2023.
Distribution: The signal crayfish is native to northwestern US, within the Columbia River Basin and areas of Washinton, Oregon, Idaho, and British Columbia. It has expanded its distribution into a variety of habitats, ranging from warm coastal waterways of the Sacramento River Delta to the sub-alpine waters of Lake Tahoe and Donner in California. It has been introduced and is established in regions of Oregon, Washington, California, Nevada, Utah, and British Columbia.
Impacts
Displacement and Loss of Native Crayfish Biodiversity
The aggressive behavior and rapid growth of invasive crayfishes make them considerable threats to native crayfish biodiversity. In fact, many studies have posed that introduced non-indigenous crayfishes are the single greatest threat to global crayfish biodiversity (Lodge et al., 2000a). In the Great Lakes region, invasive crayfishes, such as the rusty crayfish, have displaced native species from large portions of their natural ranges (Momot, 1996; Taylor and Redmer, 1996; Olden et al., 2006).

Other Aquatic Invertebrates
The impacts invasive crayfishes can have on aquatic invertebrates go far beyond crayfishes themselves. Declines in non-crayfish aquatic macroinvertebrates have been correlated with the introduction and establishment of invasive crayfishes (Charlebois and Lamberti, 1996). Invasive crayfishes have also been shown to reduce overall macroinvertebrate species richness in stream ecosystems (Stenroth and Nyström, 2003). Further, a long-term study in Wisconsin showed a considerable decline in snail densities over a period of nearly two decades following the introduction of rusty crayfish (Faxonius rusticus). The same study also reported significant declines in the abundance of multiple insects, including dragonflies and caddisflies (Wilson et al., 2004).
Declines in Native Fish Populations
Invasive crayfishes have been shown to adversely affect both native fish assemblages and recreational sport fisheries. Invasive crayfishes can cause declines in fish populations through a variety of different mechanisms. For example, invasive crayfishes can compete with native fishes for similar prey resources, and they can reduce the density of aquatic plants used by juvenile fish as cover (Wilson et al., 2004). There is also evidence that invasive crayfishes may directly affect the breeding success of some fishes by consuming their fertilized eggs (Dorn and Mittelbach, 2004).

Adverse Effects on Amphibian Breeding
Invasive crayfishes have been associated with population declines in native amphibian species around the world. One mechanism through which this can occur is breeding interference. For example, the red swamp crayfish (Procambarus clarkii), a prevalent invader in the Great Lakes, has contributed to the decline in some amphibian species through consumption of fertilized amphibian eggs (Gamradt and Kats, 1996). In ecosystems where native crayfishes are present, P. clarkii can consume amphibian eggs at a higher rate than the native crayfish species (Renai and Gherardi, 2004). Invasive crayfishes can also greatly alter resource availability, potentially influencing amphibian growth and survival (Cruz et al., 2006).

Destruction of Aquatic Plants
Aquatic plants provide shelter for fishes, amphibians, and aquatic macroinvertebrates. Crayfish have been shown to reduce macrophyte biomass and species richness through both non-consumptive and consumptive grazing (Lodge and Lorman, 1987; Wilson et al., 2004; Rosenthal et al., 2006), with invasive crayfishes consuming aquatic plant material at a faster rate than some native crayfish species. Such changes can strongly affect ecosystem structure and function, potentially resulting in the decline or displacement of other freshwater taxa within the ecosystem.
Disease Transmission
Invasive crayfishes are also a potential vector for transmitting diseases to native crayfish species. For example, crayfish plague, a devastating disease that has caused population declines and range reductions in native European crayfishes, was introduced to Europe through invasive North American crayfishes (Lodge et al., 2000a). While introduced diseases are not yet a major concern for native crayfishes in the Great Lakes, the potential for disease transmission still exists, especially from species introduced from outside North America.
Pathways

Bait
Live crayfishes are sometimes used as bait by recreational anglers. Live individuals that are left over after a long day of fishing are sometimes released directly into the water. These ‘bait-bucket’ introductions are thought to be one of the most common mechanisms by which invasive crayfishes are introduced (Ludwig and Leitch, 1996).

Aquariums
Although not typically thought of as pets, crayfishes (and other freshwater crustaceans) have become increasingly popular amongst the pet trade in recent years (Chucholl, 2013), perhaps due to their sometimes striking or unusual color patterns. The popularity of stocking crayfishes in personal aquaria, and the surge of interest among animal breeding hobbyists, have permitted the transportation of live crayfishes across state/provincial borders, and even internationally. However, some crayfishes can grow to a large size quite rapidly, and subsequently, overcrowding or aggression towards other aquatic organisms can become a problem. For this reason, crayfishes housed in aquaria are often released into nearby waterbodies. The effects of the pet trade are evident more so in Europe than in North America at present, where introduced species have transmitted the deadly crayfish plague to numerous native species (Patoka et al., 2014)

Aquatic Farming
Crayfishes are grown and sold commercially for a variety of reasons, including for food or for use as bait in recreational fishing. Aquaculture facilities supplying crayfishes to food or bait/tackle vendors are a potential introduction source for commonly cultured and potentially invasive, species such as the red swamp crayfish (Procambarus clarkii). Facilities culturing non-native crayfishes risk accidentally introducing these alien species by allowing for short-range migrations from culture ponds to nearby natural water bodies either by overland migrations or during flood events. Even facilities culturing other organisms in ponds, such as fish, can risk transporting invasive crayfishes over long distances if crayfishes make their way into the ponds and then accidentally into shipments of live animals.

Classroom Learning
Crayfishes are often used in the classroom as pets or as tools to enrich classroom learning. Some of the most memorable moments in the classroom involve studying a crayfish, but what happens to the crayfish after school? Some teachers don’t know the potential impacts of their crayfish and release them into the wild. The invasive red swamp (Procambarus clarkii) and rusty (Faxonius rusticus) crayfishes are common in biological supply kits provided to teachers for science lessons and can be accidentally released into nearby waterbodies. It is estimated that 25 percent of elementary schools in the U.S. purchase and use live crayfish in their science classes (Patton, 2011). It is important to be aware of alternatives to releasing classroom animals and plants into the wild. Even native crayfish species that are caught in the wild and brought into the classroom for learning should never be re-released into the wild.

